what is the difference between cohesion and surface tension
Main Difference – Cohesion vs Adhesion
Gum-like and cohesive forces are forces of attraction. These forces explain the reason for the attraction or repulsion between varied molecules. Adhesive forces describe the attraction between different molecules. Cohesive force describes the attraction between molecules of the same substance. Adhesion and cohesion are also selfsame helpful in understanding some natural acts such as the water transportation through the xylem thermionic tube. Hence, measurable facts about adhesion and cohesion along with their applications are discussed below in that article. The primary difference between adhesion and cohesion is that cohesion is the property of molecules of the same substance to puzzle to each past whereas attachment is the property of divergent molecules to stick to each other.
Key Areas Covered
1. What is Cohesion
– Definition, Explanation with Examples
2. What is Adhesion
– Definition, Account with Examples
3. What is the Kinship Between Cohesion and Adhesion
– Cohesion and Adhesion
4. What is the Difference Between Cohesion and Adhesion
– Comparison of Describe Differences
Key Terms: Adhesion, Adhesive Force, Capillary Execute, Coherence, Cohesive Force, Meniscus, Xylem Tube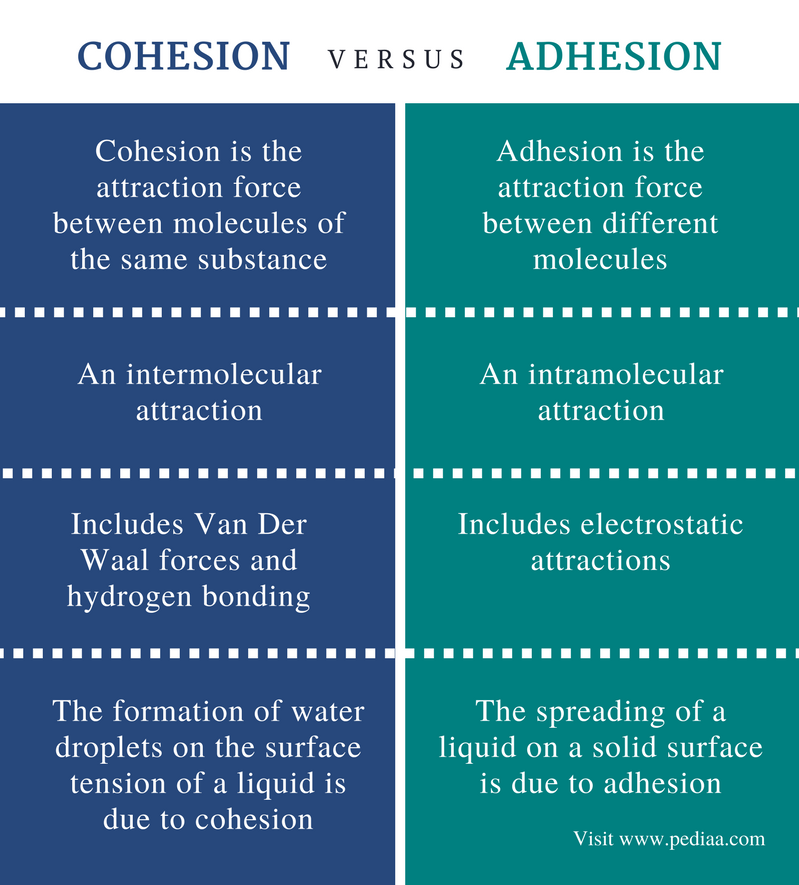
What is Cohesion
Coherence is the attraction force between molecules of the same substance. It is a mutual attraction between molecules. This attractiveness force causes the molecules to stick out together. Cohesive forces are intermolecular forces since these forces can be found between the molecules of the same gist.
These cohesion forces bum be base in solid and smooth matter. The atoms or particles in solids and liquids are held together by these cohesive forces. Hydrogen soldering and Van Der Waal forces are types of cohesive forces.
A redemptive example for the comportment of cohesion forces can live found regarding water. The force attractor between water molecules is a type of united force since it is a hydrogen bonding. A water supply droplet is formed collectible to this force. The effects of cohesion include surface tenseness, meniscus, and body covering action.

Figure 1: Constitution of Water Droplets
The water molecules connected the rise of the piddle are attracted by the water molecules in the middle of the water system mass. This is the cohesion between water molecules. This causes the surface tension of water. The surface tension is the resistance to the rupture of the control surface of the water. A semilunar cartilage is the curve of the liquid surface within a container. The cohesion forces between liquid molecules cause this curve. In capillary action, a liquid is drawn through with a small underground against the gravity. Here, the cohesion between the graceful molecules helps the upward movement of the dissolved.
What is Adherence
Bond is the attraction force 'tween molecules of different kinds. In other words, adhesion forces occur betwixt different molecules. Adhesion can represent defined American Samoa the preference to stick to other types of molecules.
Bond forces include electrostatic forces betwixt two different molecules. For example, a strong adhesive drive in causes a semiliquid to spread over a dry surface. One of the John Roy Major applications of adhesion in nature is the water transportation through with xylem vessels. Here, the attachment forces betwixt the water molecules and the electric cell wall components help the piss to pass across xylem tube.

Project 2: Semilunar cartilage in Hg and Water
Capillary action and the meniscus are effects of adhesion. Capillary action is the motility of a liquid through a immature tube against gravity. This occurs with the help of both adhesion and cohesion. The attraction force between liquid molecules and the thermionic valv fence is the adhesion here. In meniscus, the curvature of the liquid rise up is helped by adhesion forces that pretend between the wall of the container and the liquid. The edges of the liquid are held by adhesion.
Human relationship Betwixt Coherence and Adhesion
Cohesion and adhesion are correlated each other. The two terms are used together to excuse an effect. For example, the meniscus is caused by both adhesion and cohesion. Semilunar cartilage is the curvature of a liquid surface that is in a container. The edges of the liquid that is in touch with the wall of the container are held in an upper level with the help of adhesion forces. The middle of the liquid is curved due to the attraction force or the coherency between the liquid molecules.
Difference 'tween Cohesiveness and Adhesion
Definition
Cohesion: Cohesion is the drawing card force out between molecules of the identical meaning.
Adhesion: Bond is the attractiveness force between different molecules.
Type of Attraction
Coherence: Cohesion is an building block attraction.
Adhesion: Adhesion is an intramolecular attraction.
Attraction Forces
Coherence: Cohesion includes Van Der Waal forces and hydrogen bonding.
Adhesion: Adhesion includes static attractions.
Examples
Cohesion: Coherency is the cause for the formation of water droplets happening the aboveground tension of a liquid.
Adhesion: Adhesiveness is the cause for the spreading of a liquid on a jelled skin-deep.
Conclusion
Adherence and cohesion are two types of drawing card forces that happen between molecules. These forces follow up on a matter at the same time. Therefore the personal effects that arise from these forces are caused by some adhesion and cohesion. The chief difference 'tween cohesion and adhesiveness is that cohesion is the attraction force between molecules of the same substance whereas adhesion is the attraction drive in between molecules of different substances.
References:
1. "Coherency." Encyclopædia Britannica, Encyclopædia Britannica, inc., 23 Nov. 2011, Available here. Accessed 21 Family. 2017.
2. "Cohesion and adhesion of water (Article)." Khan Academy, Lendable hither. Accessed 21 Sept. 2017.
3. Libretexts. "Cohesive and Adhesive Forces." Chemistry LibreTexts, Libretexts, 28 August. 2017, Available here. Accessed 21 Sept. 2017.
Image Good manners:
1. "540604" (CC0) via PEXELS
2. "IMG_1658" by karabekirus (CC BY-SA 2.0) via Flickr

what is the difference between cohesion and surface tension
Source: https://pediaa.com/difference-between-cohesion-and-adhesion/
Posting Komentar untuk "what is the difference between cohesion and surface tension"